ASTM E1053 – Surface Time Kill Test for Viruses
Get A Testing Quote
Summary of the ASTM E1053 Method
ASTM E1053: Virucidal Efficacy of Hard Surface Disinfectants – Column Neutralization
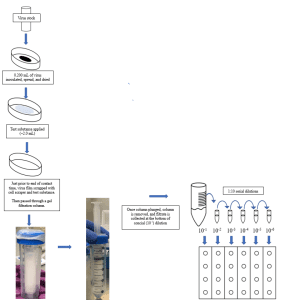
Preparation of Virus and Inoculation of Carriers:
- 100 x 15 mm Petri dishes are used for liquid and spray products, and 150 x 20 mm glass Petri dishes are used for towelettes.
- Just before inoculation of carriers, the test virus is prepared by thawing and diluting as necessary to an appropriate concentration with the Sponsor’s requested organic soil load, if applicable.
- For products intended to be marketed as a one-step disinfectant, EPA requires an organic soil load of 5% for a one-step disinfectant claim.
- Carriers are inoculated and the inoculum is spread using a sterile bent pipette tip.
- The inoculated carriers are dried at an appropriate temperature and humidity for the virus being tested.
Exposure of Virus to Test System:
- If the test substance is a spray product, the application will follow the Sponsor requested number of sprays, distance, and angle.
- If the test substance is a liquid product, an aliquot of the test substance is applied to the dried virus film.
- If the test substance is towelette, the wipe is folded appropriately and passed over the virus film. The number of passes is determined by the Study Sponsor prior to testing.
Neutralization/Infectivity Assay:
- Just prior to the contact time, the test virus film is resuspended by scraping the bottom of the carrier. The resuspended virus and test substances are aspirated and passed through a prepared gel filtration column.
- If neutralization is thought to be an issue, a chemical neutralizer may be necessary independently or via the combination of both a chemical neutralizer and gel filtration column.
- The diluent and/or filtrate then undergoes serial 10-fold dilutions in test media. These dilutions are then inoculated in quadruplicate into prepared 24 well plates.
Controls performed to Valid Assay:
- Cell Culture Control- At least 4 wells in the prepared 24-well plates are not inoculated with any virus or test substance to act as a cell culture control. This verifies that the cells are at not contaminated throughout the incubation period.
- Virus Titer Control- A volume of stock virus undergoes serial 10-fold dilutions. The dilutions are inoculated in prepared 24 well plates in quadruplicate. This confirms that the cell line used is both susceptible and permissive to the test virus.
- Plate Recovery Control- This control is performed in parallel with the test carriers. The same type of carrier used in the test is utilized for the plate recovery control and carriers are inoculated with virus inoculum in the same manner as the test carriers. After the virus has completely dried, the virus is resuspended by adding test media and scraping the carrier. The virus/media liquid is passed through a prepared gel filtration column. This filtrate undergoes serial 10-fold dilutions. These dilutions are inoculated into 24 well plates. This control is used to determine the log reduction of the product.
- Cytotoxicity Control- Test substance is applied directly to the appropriate number of carriers. At the end of the contact time, an aliquot of test media or appropriate chemical neutralizer is applied to the carriers. The bottom of the carrier is scraped and passed through a gel filtration column. This filtrate is diluted and inoculated into prepared 24 well plates. This control verifies that the test substance is not harmful (cytotoxic) to the host cell line.
- The neutralization method performed in efficacy will be used in this control.
- Test Substance Neutralization Control- This control is performed by applying the test substance to the appropriate number of carriers and harvesting the substance with test media or an appropriate chemical neutralizer. Once it is passed through a gel filtration column, the filtrate is diluted and each dilution is challenged with a low titer virus. The inoculated dilution tubes are held for at least the contact time and then plated into prepared 24 well plates. This control verifies that the test substance was properly neutralized.
- The neutralization method performed in efficacy will be used in this control.
Virucidal claims are considered additional claims in the eyes of the EPA and require the submission of bacterial base claims either prior to or concurrently with virucidal claims. Per EPA guidelines, any virucidal claims companies want to claim must be tested against. Two unique lots of products are required for testing and the hardest to inactivate viral strain must be tested with lots that are prepared at the Lower Certified Limit (LCL). The method approved for EPA submission is ASTM E1053 and can be modified for towelette, spray, and or liquid products. The requirement of a successful product for EPA is a log reduction of ≥ 3.00 log10 which translates to ≥99.9% reduction
Virucidal Efficacy Success Criteria
- At least 4.8-log10 infectious units per carrier must be recovered from the virus recovery control.
- The test product demonstrates complete inactivation of the test virus at all dilutions of the assay, or,
- if the neutralized test product causes cytotoxicity in the assay, a 3-log10 reduction past the level of cytotoxicity observed.
- Neutralization controls indicate that the selected neutralization method was successful – typically through demonstration of similar levels of cytopathic effects relative to a control.
Strengths of the ASTM E1053 Method
- Data generated will conform to U.S. EPA guidelines for efficacy data required for disinfectant claims.
- Dried viral films present a significant challenge, providing “worst case” scenario data.
- The test method may be modified to accomodate different types of test product application (spray, wipes, use-dilution) and different use conditions (soiled surfaces, varied temperatures and humidities).
Weaknesses of the ASTM E1053 Method
- Data generated for formulations against dried virus films on hard, nonporous surfaces may not translate to efficacy against viruses in suspension or other applications.
- Limits of detection can be impacted by cytotoxicity and recovery media volumes, requiring the use of a higher viral titer which places an additional level of challenge on the test products.
- Data generated for submission to the U.S. EPA may not be sufficient for regulatory agencies in other countries.
ASTM E1053 Virus Surface Time-Kill Testing according to U.S. EPA and FDA criteria can be performed at Microchem Laboratory as a simple screen (non-GLP) and under more comprehensive GLP study conditions.
