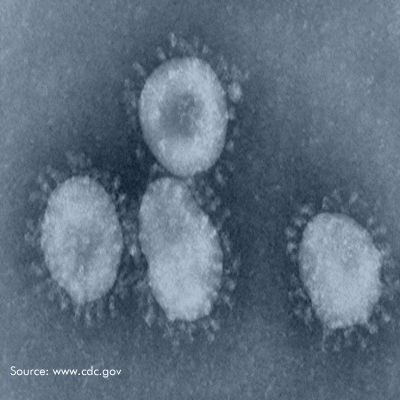
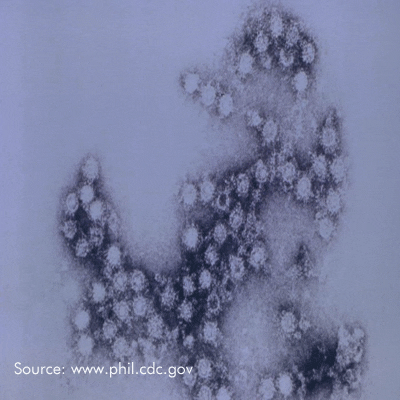
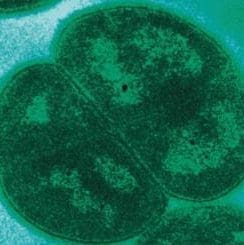
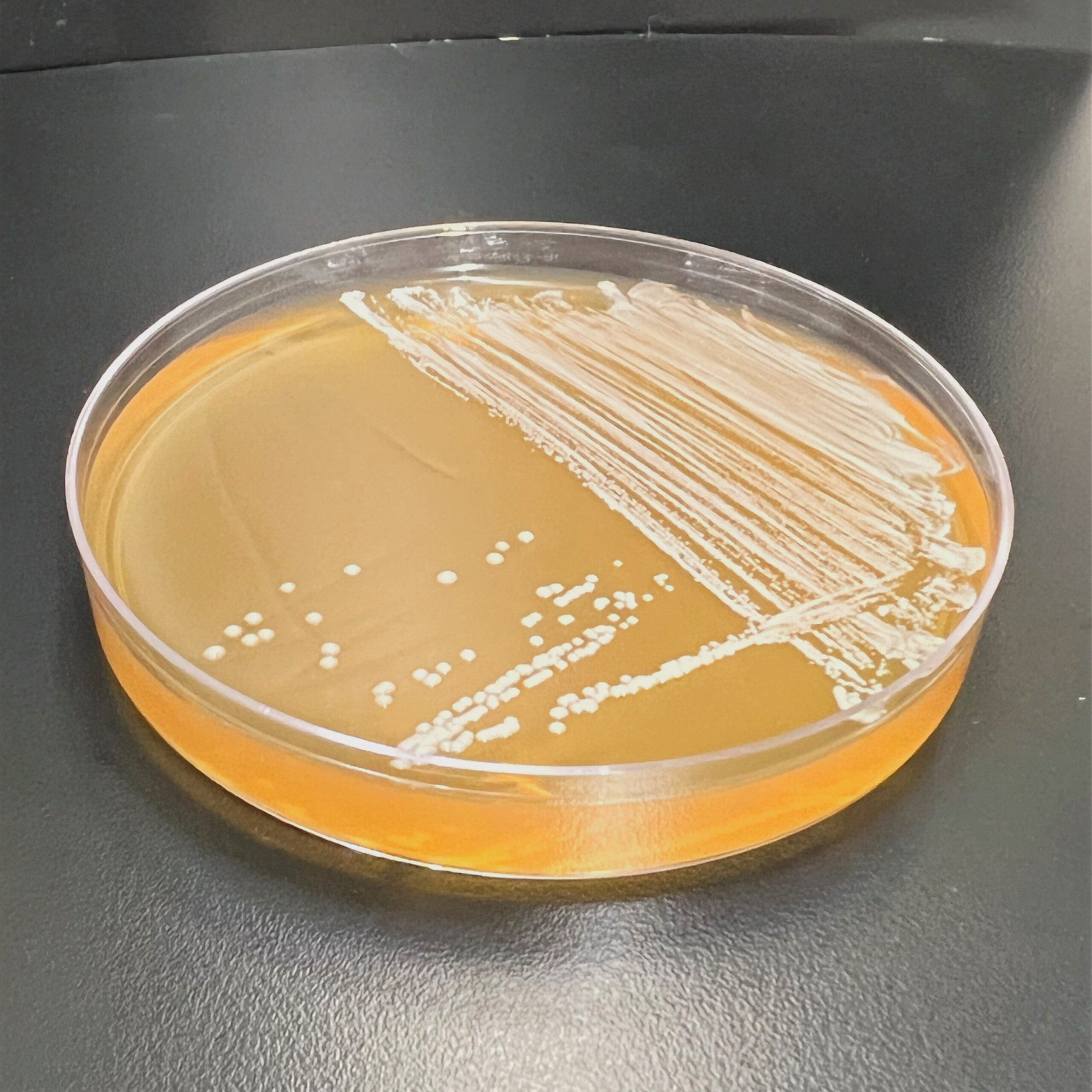
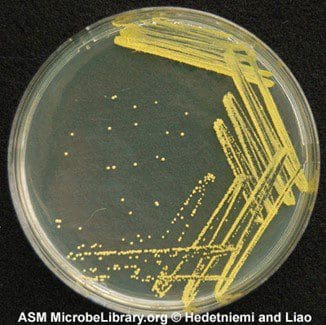
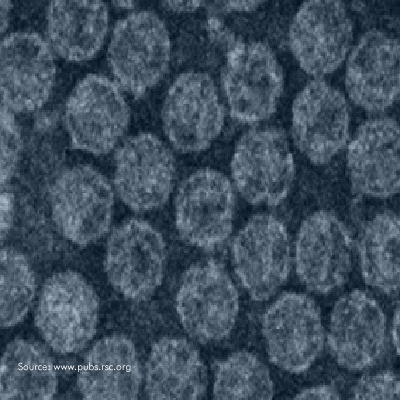
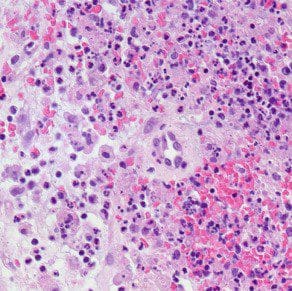
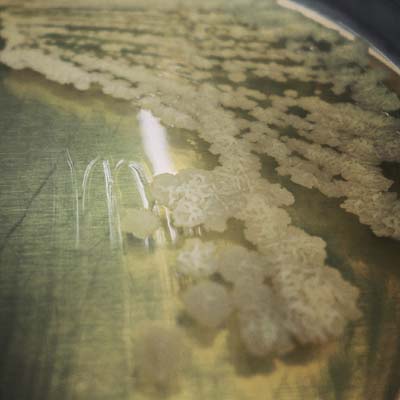
Microchem maintains an extensive collection of hundreds of microorganisms available for testing. If you have interest in a bacteria, virus, or fungus not shown, please contact us for more information.
- Buckwold, Victor E., Brigitte E. Beer, and Ruben O. Donis. “Bovine viral diarrhea virus as a surrogate model of hepatitis C virus for the evaluation of antiviral agents.” Antiviral research 60.1 (2003): 1-15.
- Ciesek, Sandra, et al. “How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its susceptibility to chemical biocides.”Journal of Infectious Diseases 201.12 (2010): 1859-1866.
- Doerrbecker, Juliane et al. “Inactivation and Survival of Hepatitis C Virus on Inanimate Surfaces.” The Journal of Infectious Diseases 204.12 (2011): 1830–1838. PMC. Web. 19 Jan. 2016.
- “Hepatitis C FAQs for Health Professionals.” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 08 Jan. 2016. Web. 19 Jan. 2016
- Lindberg, A. L. E. “Bovine viral diarrhoea virus infections and its control. A review.” Veterinary Quarterly 25.1 (2003): 1-16.
- Bidawid, S., J. Farber, and S. Sattar. 2000. Contamination of foods by food handlers: experiments on hepatitis A virus transfer to food and its interruption. 66: 2759-2763.
- Bowen, G. and M. McCarthy. 1983. Hepatitis A associated with a hardware store water fountain and a contaminated well in Lancaster County, Pennsylvania. American Journal of Epidemiology. 117: 695-705.
- Cuthbert, J. 2001. Hepatitis A: old and new. Clinical Microbiology Reviews. 14: 38-58.
- Doebbling, B., N. Li, and R. Wenzel. 1993. An outbreak of hepatitis A among health care workers: risk factors for transmission. American Journal of Public Health. 83: 1679-1684.
- Farthing, M. 1989. Viruses and the Gut. Published by Smith, Kline, & French, Garden City, United Kingdom.
- Matthew, E. et al. 1973. A major epidemic of infectious hepatitis in an institution for the mentally retarded. American Journal of Epidemiology. 98: 199-215.
- Mbithi, J., S. Springthorpe, and S. Sattar. 1990. Chemical disinfection of hepatitis A on environmental surfaces. Applied and Environmental Microbiology. 56: 3601-3604.
- Mbithi, J., S. Springthorpe, and S. Sattar. 1991. Effect of relative humidity and air temperature in survival of hepatitis A virus on environmental surfaces. Applied and Environmental Microbiology. 57:1394-1399.
- Mbithi, J., S. Springthorpe, J. Boulet, and S. Sattar. 1992. Survival of hepatitis A virus on human hands and its transfer on contact with animate and inanimate surfaces. Journal of Clinical Microbiology. 30:757-763.
- Mbithi, J., S. Springthorpe, and S. Sattar. 1993. Comparative in vivo efficiencies of hand-washing agents against hepatitis A virus (HM-175) and poliovirus type 1(Sabin). Applied and Environmental Microbiology. 57:1394-1399.
- Vernon, A., C. Schable, and D. Francis. 1982. A large outbreak of hepatitis A in a day-care center: association with non-toilet-trained children and persistence of IgM antibiody to hepatitis A virus. American Journal of Epidemiology. 115: 325-331.
- Wheeler, C. et al. 2005. An outbreak of hepatitis A associated with green onions. The New England Journal of Medicine. 353: 890-897.
- Boone, Stephanie A., and Charles P. Gerba. “Significance of fomites in the spread of respiratory and enteric viral disease.” Applied and Environmental Microbiology 73.6 (2007): 1687-1696.
- Butz, Arlene M., et al. “Prevalence of rotavirus on high-risk fomites in day-care facilities.” Pediatrics 92.2 (1993): 202-205.
- Clark, Benjamin, and Mike McKendrick. “A review of viral gastroenteritis.”Current opinion in infectious diseases 17.5 (2004): 461-469.
- Lloyd-Evans, N., V. S. Springthorpe, and S. A. Sattar. “Chemical Disinfection of Human Rotavirus-Contaminated Inanimate Surfaces.” The Journal of Hygiene 97.1 (1986): 163–173. Print.
- “Rotavirus.” Immunization, Vaccines, and Biologicals. World Health Organization, 13 Apr. 2015. Web.
- Baker, David G. “Natural pathogens of laboratory mice, rats, and rabbits and their effects on research.” Clinical microbiology reviews 11.2 (1998): 231-266.
- Eterpi, M., G. McDonnell, and V. Thomas. “Disinfection efficacy against parvoviruses compared with reference viruses.” Journal of Hospital Infection73 (2009): 64e70.
- Grekova, Svitlana et al. “Activation of an Antiviral Response in Normal but Not Transformed Mouse Cells: A New Determinant of Minute Virus of Mice Oncotropism .” Journal of Virology 84.1 (2010): 516–531. PMC. Web. 18 Jan. 2016
- Marchini, Antonio et al. “Oncolytic Parvoviruses: From Basic Virology to Clinical Applications.” Virology Journal 12 (2015): 6. PMC. Web. 18 Jan. 2016.
- D’Souza, D.H. and X. Su. 2010. Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS-2 bacteriophage. Foodborne Pathogens and Disease. 7(3): 319-326.
- Malik, Y.S. et al. 2006. Disinfection of fabrics and carpets artificially contaminated with calicivirus: relevance in institutional and healthcare centres. Journal of Hospital Infection. 63(2): 205-210.
- Park, G.W. et al. 2010. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. Journal of Food Protection. 73(12): 2232-2238.
- Whitehead, K. and K.A. McCue. 2010. Virucidal efficacy of disinfectant actives against feline calicivirus, a surrogate for norovirus, in a short contact time. American Journal of Infection Control. 38(1): 26-30.
- Barker, J. D. Stevens, and S.F. Bloomfield. 2001. Spread and prevention of some common viral infections in community facilities and domestic homes. Journal of Applied Microbiology. 91: 7-21.
- Barker, J., I.B. Vipond, and S.F. Bloomfield. 2004. Effects of cleaning and disinfections in reducing the spread of norovirus contamination via environmental surfaces. Journal of Hospital Infection. 58: 42-49.
- Becker, K.M. et al. 2000. Transmission of Norwalk virus during a football game. The New England Journal of Medicine. 343(17): 1223-1227.
- Bidawid, S. et al. 2004. Norovirus cross-contamination during food handling and interruption of virus transfer by hand antisepsis: experiments with feline calicivirus as a surrogate. Journal of Food Protection. 67(1): 103-107.
- Boone, S.A. and C.P. Gerba. 2007. Significance of fomites in the spread of respiratory and enteric viral disease. Applied and Environmental Microbiology. 73(6): 1687-1696.
- Bright, K.R., S.A. Boone, and C.P. Gerba. 2010. Occurrence of bacteria and viruses on elementary classroom surfaces and the potential role of classroom hygiene in the spread of infectious diseases. The Journal of School Nursing. 26(1): 33-41.
- Cannon, J.L. et al. 2006. Surrogate for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. Journal of Food Protection. 69(11): 2761-2765.
- Costantini, V. et al. 2006. Human and animal enteric caliciviruses in oysters from different coastal regions of the United States. Applied and Environmental Microbiology. 72(3): 1800-1809.
- Doultree, J.C. et al. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. Journal of Hospital Infection. 41(1): 51-57.
- Fankem, S.L. et al. 2009. Assessment of disinfectant performance in chicken cages using coliphages. Food and Environmental Virology. 1: 155-160.
- Friedman, D.S. et al. 2005. An outbreak of norovirus gastroenteritis associated with wedding cakes. Epidemiology and Infection. 133(6): 1057-1063.
- Glass, R.I., U.D. Parashar, and M.K. Estes. 2009. Norovirus gastroenteritis. New England Journal of Medicine. 361: 1776-1785.
- Jimenez, L. and M. Chiang. 2006. Virucidal activity of a quaternary ammonium compound disinfectant against feline calicivirus: a surrogate for norovirus. American Journal of Infection Control. 34(5): 269-273.
- Maillard, J.-Y. et al. 1994. Effect of biocides on MS2 and K coliphages. Applied and Environmental Microbiology. 60(6): 2205-2206.
- Malek, M. et al. 2009. Outbreak of norovirus infection among river rafters associated with packaged delicatessen meat, Grand Canyon, 2005. Clinical Infectious Diseases. 48: 31-37.
- Malik, Y.S., S. Maherchandani, and S.M. Goyal. 2006. Comparative efficacy of ethanol and isopropanol against feline calicivirus, a norovirus surrogate. American Journal of Infection Control. 34(1): 31-35.
- Radford, A.D. et al. 2007. Feline calicivirus. Veterinary Research. 38: 319-335.
- Said, M.A., T.M. Perl, and C.L. Sears. 2008. Gastrointestinal flu: norovirus in health care and long-term care facilities. Clinical Infectious Disease. 47: 1202-1208.
- Sivapalasingam, S. et al. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. Journal of Food Protection. 67(10): 2342-2353.
- Widdowson, M.-A. et al. 2004. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus – United States, 2002. The Journal of Infectious Diseases. 190: 27-36.
- Wobus, C.E., L.B. Thakray, and H.W. Virgin IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. Journal of Virology. 80(11): 5104-5112.
- Abad, F., R. Pinto, and A. Bosch. 2006. Disinfection of human enteric viruses on fomites. FEMS Microbiology Letters. 156: 107-111.
- Abad, F., R. Pinto, and A. Bosch. 1994. Survival of enteric viruses on environmental fomites. Applied and Environmental Microbiology. 60: 3704-3710.
- Bailly, J. et al. 1991. Activity of glutaraldehyde at low concentrations (less than 2%) against poliovirus and its relevance to gastrointestinal endoscope disinfection procedures. Applied and Environmental Microbiology. 57: 1156-1160.
- Centers for Disease Control and Prevention. Poliomyelitis: Epidemiology and Prevention of Vaccine-Preventable Diseases. The Pink Book: Course Textbook – 12th Edition Second Printing, May 2012.
- Chumakov, K. et al. 2007. Vaccination against polio should not be stopped. Nature Reviews Microbiology. Advance Online Publication, published online 29 October 2007; doi:10.1038/nrmicro1769.
- Enriquez, C., C. Hurst and C. Gerba. 1995. Survival of enteric adenoviruses 40 and 41 in tap, sea, and waste water. Water Research. 29: 2548-2553.
- Heaselgrave, W. et al. 2006. Solar disinfection of poliovirus and Acanthamoeba polyphaga cysts in water – a laboratory study using simulated sunlight. Letters in Applied Microbiology. 43: 125-130.
- Hurst, C., W. Benton, and A. McClellan. 1989. Thermal and water source effects upon the stability of enteroviruses in surface freshwaters. Canadian Journal of Microbiology. 35: 474-480.
- Kew, O. et al. 2004. Circulating vaccine-derived polioviruses: current state of knowledge. Bulletin of the World Health Organization. 82: 16-23. Kim-Farley, R. et al. 1984. Outbreak of paralytic poliomyelitis, Taiwan. The Lancet. 324: 1322-1324.
- Meng, Q.S. and C. Gerba. 1996. Comparative inactivation of enteric adenoviruses, poliovirus and coliphages by ultraviolet irradiation. Water Research. 30: 2665-2668.
- Oostvogel, P. et al. 1994. Poliomyelitis outbreak in an unvaccinated community in the Netherlands. The Lancet. 344: 665-670.
- Poyry, T., M. Stenvik and T. Hovi. 1988. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Applied Environmental Microbiology. 54: 371-374.
- Rutala, W. et al. 2000. Antimicrobial activity of home disinfectants and natural products against potential human pathogens. Infection Control and Hospital Epidemiology. 21: 33-38.
- Shin, G-A. and M. Sobsey. 2003. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Applied Environmental Microbiology. 69: 3975-3978.
- Strauss, J.H. and E.G. Strauss. Viruses and Human Disease. Elsevier Academic Press, Burlington, MA. 2008.
- Ansaldi, F. et al. 2004. SARS-CoV, influenza A and syncytial respiratory virus resistance against common disinfectants and ultraviolet radiation. Journal of Preventative Medicine and Hygiene. 45: 5-8.
- Boone, S.A. and C.P. Gerba. 2007. Significanace of fomites in the spread of respiratory and enteric viral disease. Applied and Environmental Microbiology. 73(6): 1687-1696.
- Hall, C., R. Douglas, and J. Geiman. 1980. Possible transmission by fomites of respiratory syncytial virus. 141: 98-102.
- Gwaltney, J. and J. Hendley. 1982. Transmission of experimental rhinovirus infection by contaminated surfaces. American Journal of Epidemiology. 116: 828-833.
- Hendley, J., R. Wenzel, and J. Gwaltney. 1973. Transmission of rhinovirus colds by self-inoculation. The New England Journal of Medicine. 288: 1361-1364.
- Hendley, J., L. Mika, and J. Gwaltney. 1978. Evaluation of virucidal compounds for inactivation of rhinovirus on hands. Antimicrobial Agents and Chemotherapy. 14: 690-694.
- Lessler, J. et al. 2009. Incubation periods of acute respiratory viral infections: a systematic review. The Lancet Infectious Diseases. 9: 291-300.
- Longtin, J. et al. 2010. Rhinovirus outbreaks in long-tern care facilities, Ontario, Canada. Emerging Infectious Diseases. 16: 1463-1465.
- Louie, J. et al. 2005. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clinical Infectious Diseases. 41: 262-265.
- Sattar, S. et al. 1987. Survival of human rhinovirus type 14 dried onto nonporous inanimate surfaces: effect of relative humidity and suspending medium. Canadian Journal of Microbiology. 33: 802-806.
- Sattar, S. et al. 1993. Chemical disinfection to interrupt transfer of rhinovirus type 14 from environmental surfaces to hands. Applied and Environmental Microbiology. 59: 1579-1585.
- Strauss, J.H. and E.G. Strauss. Viruses and Human Disease. Elsevier Academic Press, Burlington, MA. 2008.
- Turner, R., J. Fuls, and N. Rodgers. 2010. Effectiveness of hand sanitizers with and without organic acids for removal of rhinovirus from hands. Antimicrobial Agents and Chemotherapy. 54: 1363-1364.
- Goodger, W. J., et al. “Economic impact of an epizootic of bovine vesicular stomatitis in California.” Journal of the American Veterinary Medical Association 186.4 (1985): 370.
- SHIRAI, Junsuke, et al. “Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses.”Journal of Veterinary Medical Science 62.1 (2000): 85-92.
- United States of America. USDA. Animal and Plant Health Inspection Service. Vesicular Stomatitis Fact Sheet. Animal and Plant Health Inspection Service, May 2012. Web.
| Virus | Bovine Viral Diarrhea Virus | Virus | Hepatitis C Virus |
| Structure | Enveloped | Structure | Enveloped |
| Family | Flaviviridae | Family | Flaviviridae |
| Primary Host | Cattle | Primary Host | Humans |
| Disease(s) Caused | Bovine Viral Diarrhea | Disease(s) Caused | Hepatitis C |
| Symptoms | Fever, lethargy, loss of appetite, oral lesions, diarrhea, decreased milk production | Symptoms | Fatigue, nausea, muscle aches |
| Potential Complications | Secondary bacterial infections | Potential Complications | Liver cirrhosis, liver cancer |
| Transmission Mode | In utero, contact with secretions from persistently infected animals | Transmission Mode | Blood-borne |
| Sites of Community Outbreaks | Farms, ranches | Sites of Community Outbreaks | Healthcare settings, drug users |
Importance of Bovine Viral Diarrhea Virus
Bovine Viral Diarrhea Virus (BVDV) is an enveloped member of the Flaviviridae family. BVDV is a major pathogen for cattle and also serves as the U.S. EPA approved surrogate for Human Hepatitis C.
There are two types of BVDV, noncytopathic and cytopathic. The noncytopathic type is able to persist in the body by suppressing the immune response. This type is usually non-symptomatic in healthy adults, but can leave the animal more susceptible to other infections as a result of the immune suppression. The cytopathic type is responsible for mucosal disease, a fatal condition. Mucosal disease causes lesions on the intestinal walls, resulting in severe diarrhea and dehydration. Bacterial infection of the lesions contributes to mortality.
If a pregnant cow is infected with BVDV in the first trimester, the fetus’ developing immune system can ignore the virus, thus becoming persistently infected (PI). These animals, if they survive to birth, are the major source of transmission in a herd, shedding virus throughout their lifespan. Because the noncytopathic type suppresses the immune system, PI animals are more likely to become ill. Mucosal disease can arise in PI animals via either mutation of the persistent strain into the cytopathic type, or by secondary infection. This typically happens before the animal is 2 years old.
BVDV is accepted by the U.S EPA as a surrogate for disinfectant efficacy testing against Human Hepatitis C virus (HCV). A surrogate is necessary because HCV is difficult to propagate reproducibly in cell culture. BVDV works well as a surrogate due to a high degree of genomic similarity between the two viruses. Their genomes have been found to code for functionally identical proteins that can serve as targets for inactivation. Both viruses are of similar size and both are enveloped, indicating similar susceptibility to disinfection. In addition, both viruses establish persistent infections in their hosts at a similar frequency.
Importance of Hepatitis C
HCV is an enveloped member of the Flaviviridae family. HCV causes hepatitis C, a disease that affects the liver.
Infection with HCV can cause either an acute or chronic infection. About 15% of cases are acute, with the remainder being chronic. Acute cases typically cause mild symptoms such as fatigue, nausea, and muscle aches. The liver is not usually involved. Chronic infections can result in liver cirrhosis or cancer. Co-infections and excessive alcohol consumption greatly increases the chances of this happening.
HCV is blood-borne. Transmission can occur in several ways. Intravenous drug use is a major vector. Tattoos performed with a non sterile needle or contaminated dye is another way that HCV can be spread. Healthcare settings can also pose a risk when reusable equipment is improperly sterilized, or when accidental needle sticks occur.
Importance of Disinfection: Survival of Bovine Viral Diarrhea Virus and Hepatitis C on Surfaces and Potential for Transmission via Fomites
BVDV is spread primarily through direct contact with a PI animal. Transmission can also occur via surfaces contaminated with bodily fluids from a PI animal.
HCV has been shown to survive and remain infectious for up to 1 week. Infected surfaces, such as needles, can easily transmit HCV, as can contaminated suspensions.
Both BVDV and HCV are susceptible to a range of disinfectants, as with most enveloped viruses.
References
| Virus | Hepatitis A Virus |
| Structure | Non-enveloped |
| Family | Picornaviridae |
| Host(s) | Humans |
| Disease(s) Caused | Acute liver disease |
| Symptoms | Juandice, nausea, fever, lethargy, abdominal pain |
| Potential Complications | Acute liver failure |
| Transmission Mode | Fecal-Oral: Person-to-person contact; foodborne; waterborne; contaminated fomites |
| Sites of Community Outbreaks | Daycare centers; restaurants |
Importance of Hepatitis A Virus
Hepatitis A virus is one of the five major hepatitis viruses, along with B, C, D, and E. Hepatitis viruses A and E are spread via the fecal-oral route, unlike B, C, and D, which are bloodborne pathogens. Upon ingestion of infectious hepatitis A virus, an incubation period follows ranging from 10 to 50 days. Infected persons may exhibit symptoms, such as nausea/vomiting, abdominal pain, anorexia, and headache. Some develop the telltale yellowing of the skin and eyes (jaundice) associated with damage caused to the liver during illness. However, a number of infected persons remain asymptomatic or do not develop jaundice; this tendency has been correlated to age in several outbreak studies (3).
Outbreaks of hepatitis A virus illness have been associated with settings characterized by a high frequency of person-to-person contact including hospitals, daycare centers, and institutional centers (4, 6, 11). A number of outbreaks have also been associated with restaurants, water, shellfish, and crops (2, 12).
The Importance of Disinfection: Survival of Hepatitis A Virus on Surfaces and Transmission Potential via Fomites
Following viral replication in hepatocytes, hepatitis A virus is released via the bile duct into the intestinal tract and into the environment with fecal excrement. Infected persons can shed anywhere from 5-log10 to 11-log10 viruses per gram of feces (5). Deposition of the virus onto the hands or fomites can occur with the release of feces with survival and subsequent transfer in the environment dependent on temperature, humidity, and other factors. In contrast to related enteroviruses, such as poliovirus, hepatitis A remained active under conditions of low relative humidity when dried on stainless steel disks in the presence of fecal material with substantial reductions observed at high relative humidities of 80 ± 5%; lower temperatures facilitated enhanced survival similar to poliovirus-1 (8). A substantial loss of hepatitis A viral infectivity follows within one hour of inoculation onto hands and stainless steel disks; however, transfer efficiency from fingerpads to disks was still demonstrated after four hours (9).
The implications of hepatitis A virus transfer from hands are of great importance, particularly when the objects are food items that will not be cooked or heat-treated, such as raw produce. Approximately 9% of hepatitis A viruses inoculated onto the fingerpads of volunteers were transferred to lettuce (representing from 1 to 1,300 infectious viral units), demonstrating a possible scenario for the initiation of infection in potential human hosts (1). However, these transfer efficiencies were drastically reduced by the use of topical agents prior to the handling of the lettuce.
The tough and hardy structure of hepatitis A virus grants a unique resistance to inactivation by many chemical disinfectants. Following a 1-minute contact time, only 2% glutaraldehyde, a quaternary ammonium compound formulation containing 23% HCl, and sodium hypochlorite (>5,000 ppm free chlorine) reduced dried film hepatitis A viral titers by > 3-log10 (>99.9%), where phenolics, iodides, alcohols, and various acids proved ineffective (7). This resistance has also been demonstrated for a number of handwashing agents. A number of formulations have proven more efficacious against poliovirus-1 than hepatitis A virus, although infectious viruses remaining on the hands after washing were highlighted as an ongoing issue of concern (10).
References
| Virus | Human Rotavirus |
| Structure | Non-enveloped |
| Genome | Double stranded RNA |
| Family | Reoviridae |
| Primary Host | Humans |
| Disease(s) Caused | Gastroenteritis |
| Symptoms | Nausea, vomiting, diarrhea, fever |
| Potential Complications | Severe dehydration |
| Transmission Mode | Fecal-oral, contact with contaminated fomites |
| Sites of Community Outbreaks | Hospitals, daycares, schools, nursing homes |
Importance of Human Rotavirus
Human rotavirus is a non-enveloped member of the Reoviridae family. Seven serogroups for this virus exist. Serogroup A is responsible for the majority of infections with B and C responsible for sporadic outbreaks.
Rotavirus is a significant cause of severe diarrhea in infants and young children around the world. In 2008, the World Health Organization estimated that 453,000 children died as a result of rotavirus infection, accounting for around 5% of all child deaths. Most infections occur in children aged 6 months to 2 years old.
Rotavirus infection results in watery diarrhea and vomiting, along with fever and abdominal pain. During this period, dehydration can occur if fluids are not adequately replaced. Symptoms generally persist for 3-8 days.
Importance of Disinfection: Survival of Rotavirus on Surfaces and Potential for Transmission via Fomites
As a non-enveloped virus, rotavirus is resistant to environmental factors such as humidity and temperature. Rotavirus has been found to remain infectious on a metal surface for at least 2 months at a variety of temperatures and humidity levels. Rotavirus can be difficult to inactivate. In one study, only 9 out of 27 disinfectants tested were shown to demonstrate a 99.9% reduction in rotavirus dried onto stainless steel carriers. These disinfectants included 2% glutaraldehyde, some quaternary ammonium compounds combined with either alcohol or acid, phenols with a surfactant, iodine with at least 10,000 ppm free iodine, and chlorine based disinfectants with at least 20,000 ppm free chlorine.
Rotavirus is transmitted via the fecal-oral route. The virus is shed in feces for up to 3 days after the patient has recovered from infection. A key way that rotavirus is spread is when someone touches a contaminated surface and then touches their mouth. Surfaces likely to be contaminated include infected individuals’ hands, doorknobs, sinks, toilets, and faucets. In daycares, up to 19% of surfaces were found to be contaminated with rotavirus during an outbreak.
There are currently two vaccines available for rotavirus. These vaccines became available in 2006 worldwide and have proven effective, reducing hospitalizations and deaths significantly. However, unvaccinated people are still at risk, making effective disinfection key to disease prevention.
References
| Virus | Measles Virus |
| Structure | Enveloped |
| Genome | Single stranded RNA, negative sense |
| Family | Paramyxoviridae |
| Primary Host | Humans |
| Disease(s) Caused | Measles |
| Symptoms | Four-day fevers, cough, head cold, fever, sneezing, conjuctivitis, rash |
| Potential Complications | Diarrhea, pneumonia, brain inflammation, blindness |
| Transmission Mode | Contact with infected secretions, close personal contact with infected person |
| Sites of Community Outbreaks | Schools, daycares, churches |
Importance of Measles Virus
Measles virus is an enveloped member of the Paramyxoviridae family. Its genome consists of a single, negative sense strand of RNA.
Measles virus is responsible for the disease known as measles. Measles presents as a high fever with cough, coryza, and conjunctivitis. After 2-4 days of symptoms, the characteristic red rash appears. The rash spreads over the face and torso, increasing in severity for 2-3 days. After 3-7 days, the rash begins to recede. The fever usually remains for the first few days of the rash, and the cough can persist for up to a week.
Complications of measles can include bronchitis and pneumonia, caused either by the measles virus itself or by co-infection with another pathogen. Measles can also cause encephalitis if the nervous system is infected. Blindness can occur if the patient experiences a secondary ocular infection or is vitamin A deficient, since measles reduces the levels of vitamin A in the blood.
Importance of Disinfection
Measles is highly contagious. Approximately 90% of those susceptible who come into close contact with a measles patient will develop the disease. Patients are contagious for the 4 days prior to and 4 days after the appearance of the rash. During this time, the virus is shed in nasal and throat secretions.
Measles is spread primarily through the airborne route. Measles has been shown to be able to survive suspended in air for at least one hour, and on surfaces for up to 2 hours.
A vaccine for measles was introduced in 1963. The vaccine is responsible for a 79% decrease in deaths from 2000 to 2014 worldwide.
Since 2000, measles has been declared eliminated from the United States. However, cases of measles still appear every year when travelers bring the virus back from countries where it is still endemic. Outbreaks also still occur among non-vaccinated populations.
As an enveloped virus, measles virus is susceptible to a wide range of disinfectants. UV room disinfection may also be effective in preventing airborne transmission.
References
Bloch, Alan B., et al. “Measles outbreak in a pediatric practice: airborne transmission in an office setting.” Pediatrics 75.4 (1985): 676-683.
“Measles.” World Health Organization. World Health Organization, Nov. 2015. Web. 20 Jan. 2016.
“Measles (Rubeola).” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 10 Aug. 2015. Web. 20 Jan. 2016.
Orenstein, Walter A., Robert T. Perry, and Neal A. Halsey. “The clinical significance of measles: a review.” Journal of Infectious Diseases189.Supplement 1 (2004): S4-S16.
Reed, Nicholas G. “The History of Ultraviolet Germicidal Irradiation for Air Disinfection.” Public Health Reports 125.1 (2010): 15–27. Print.
| Virus | Minute Virus of Mice |
| Structure | Non-enveloped |
| Genomes | Single stranded DNA |
| Family | Parvoviridae |
| Primary Host | Mice |
| Disease(s) Caused | None |
| Symptoms | None |
| Potential Complications | Possible organ damage if infection occurs in utero or shortly after birth, interference with research |
| Transmission Mode | Contact with nasal secretions and contaminated fomites |
| Sites of Community Outbreaks | Mouse colonies |
Importance of Minute Virus of Mice
Minute virus of mice (MVM) is a small non-enveloped virus belonging to the Parvoviridae family. MVM’s natural hosts are laboratory and wild mice, but experimental infection of rats and hamsters has been successful.
MVM infection does not typically cause symptoms in healthy adult mice. However, infection occuring during fetal development or shortly after birth can result in severe damage to multiple organs.
There are two common strains of MVM: a prototype strain, MVM(p), and an immunosuppressive strain, MVM(I). MVM(p) infects fibroblasts, whereas MVM(I) infects T lymphocytes.
MVM is of special concern for laboratory mouse colonies. Because it is asymptomatic in adults, detecting it is difficult. Infection with MVM(I) will alter T lymphocyte activity, which can potentially interfere with research focusing on the immune system.
MVM has also been shown to have the ability to suppress cancer. MVM replicates most efficiently in cancerous cells, which lack the ability to form a type 1 interferon antiviral response. Infection triggers cell cycle arrest and the DNA damage response pathway, as well as a general host immune response that targets infected cells. These effects of MVM infection can potentially interfere with research that involves tumor formation in mice.
Importance of Disinfection: Survival of Minute Virus of Mice of Surfaces and Potential for Transmission via Fomites
MVM, as with most parvoviruses, is highly contagious and able to persist on environmental surfaces for long periods of time.
MVM is spread primarily through infected urine and feces, as well as nasal secretions. Contact with infected fomites, such as food and water bowls or bedding, can also spread MVM.
As a parvovirus, MVM is resistant to a range of disinfectants. A study showed that at a contact time of 10 minutes, 70% ethanol, 7.5% hydrogen peroxide and 0.05% quaternary ammonium were all ineffective against MVM. MVM was shown to be susceptible to 2% glutaraldehyde, 0.55% ortho-phthaldehyde, 0.2% peracetic acid, and 2500 ppm sodium hypochlorite.
References
| Virus | Human norovirus |
Feline calicivirus (U.S.-EPA human norovirus surrogate) |
| Structure | Non-enveloped | Non-enveloped |
| Family | Caliciviridae | Caliciviridae |
| Host(s) | Humans | Cats |
| Disease(s) Caused | Acute gastroenteritis | Respiratory illness, pneumonia, fever |
| Symptoms | Nausea, vomiting, diarrhea, abdominal cramping | Runny nose/eyes, sneezing, fever, mouth ulcers |
| Potential Complications | Severe dehydration | Pneumonia, lameness |
| Transmission Mode |
Environmental: Person-to-Person: Fomite: contaminated surfaces |
Cat-to-cat contact: eye/nasal/mouth fluids Fomite transmission: |
| Sites of Community Outbreaks* | Cruise ships Restaurants/Catered events Recreational sites Hospitals Nursing homes Schools/Daycare centers |
Animal shelters Pet stores Veterinary clinics |
Importance of Human Norovirus
Human noroviruses are a highly infectious group of viral pathogens that cause epidemics of acute gastroenteritis annually in the United States and worldwide. Illness is characterized by a rapid onset of vomiting and/or diarrhea, nausea, and abdominal pain that is short-lived, generally lasting from 24 to 72 hours. However, noroviruses may be shed prior to the onset of symptoms and in feces for several weeks after the illness has subsided (12).
Norovirus outbreaks generally occur in locations conducive to person-to-person spread including schools, hospital wards, and cruise ships (1, 6, 18, 20). Epidemics have also been associated with events such as weddings, rafting trips, and sporting events (3, 11, 15), in addition to consumption of raw produce, shellfish, and deli food items (8, 15, 19).
The Use of Surrogate Viruses for Human Norovirus Studies and Efficacy Testing
Despite their prevalence in the human population, noroviruses have proven difficult to successfully propagate in a cell culture system. Therefore, MS-2 bacteriophage, feline calicivirus, and more recently murine norovirus have been employed as models for human noroviruses in virucidal efficacy studies.
MS-2 coliphage is a virus that infects “male” Escherichia coli cells exhibiting the F pilus on their surface. MS-2 and human noroviruses are structurally similar non-enveloped viruses; each are comprised of icosahedral nucleocapsids exhibiting the same triangulation number (T=3). MS-2 coliphages are also exceptionally hardy and environmentally resistant. They are relatively simple to cultivate and purify, with high viral titers readily achieved in the laboratory setting under suitable conditions. Virucidal efficacy evaluations that utilize MS-2 coliphage as a surrogate for human norovirus or other non-enveloped viruses also yield rapid test results (18 to upwards of 48 hours after assay initiation relative to 5 to 7 days for the feline calicivirus surrogate). Viral plaques are visualized on an E. coli host lawn, and assay results are generally presented in units of plaque-forming units (PFU) per unit volume or per carrier. Biocidal efficacy evaluations using MS-2 as a non-enveloped mammalian virus surrogate have been published in the literature including suspension time-kill tests and surface disinfectant testing (10, 14).
Feline calicivirus (FCV) and human noroviruses belong to the same viral family, Caliciviridae, although to different genera (Vesivirus and Norovirus, respectively). FCV and human noroviruses are therefore highly comparable in terms of size and structure. The higher degree of phylogenetic relativity in conjunction with an established and straightforward cell culture assay system led to the establishment of feline calicivirus as the U.S.-EPA recommended human norovirus surrogate for virucidal efficacy testing in 2005. The host cell line for feline calicivirus is Crandell-Rees Feline Kidney (CRFK), which is readily established and maintained by way of standard sub-culturing techniques. Although the aforementioned assay period of 5 to 7 days is longer than that of MS-2, the data generated from the U.S. EPA-required TCID50 (Tissue Culture Infectivity Dose at the 50% Endpoint) assay is consistent given the proper performance of required study controls (examples: Virus Control and Cytotoxicity). Viral and toxicity titers are then assessed using an established statistical method (examples: Reed-Muench or Spearman-Karber). Feline caliciviruses have been employed in many studies evaluating each of the disinfectant classes, including alcohols, halogens, quaternary-ammonium compounds, and glutaraldehydes (9, 13, 16).
Similar to feline calicivirus, murine norovirus (MNV) belongs to the same viral family as the human noroviruses. Unlike feline calicivirus, however, MNV is grouped within the genus Norovirus, thus sharing even greater phylogenetic relatedness to the human noroviruses. In addition, MNV and human noroviruses are demonstrated enteric pathogens of mice and humans, respectively, whereas feline calicivirus infects via nasal, oral, and conjunctival routes (17). The cell culture assay for murine norovirus (MNV-1) utilizes a mouse macrophage host cell line performed either using a plaque assay or TCID50 assay (21). MNV has become increasingly used as a surrogate for human norovirus in virucidal efficacy evaluations as well.
Direct assessment of anti-viral efficacy against human noroviruses would be most ideal. However, a straightforward cell culture system allowing for detection of infectious noroviruses remains elusive. While each of the viral surrogates for human norovirus has associated pros and cons, they continue to serve as viable research models for efficacy testing. The U.S. EPA has also maintained the use of feline calicivirus as an acceptable model for human norovirus disinfectant label claims.
The Importance of Disinfection: Survival of Human Norovirus Surrogates on Surfaces and Transmission Potential via Fomites
The transmission route of human noroviruses is generally described as fecal-oral. During the short-lived bout of gastroenteritis, an infected person may shed upwards of 11-log10 viruses per gram of feces (5), usually by loose bowels or outright diarrhea. High numbers of viruses are also expelled from the acute projectile vomiting episodes commonly associated with human norovirus infections (1). During the course of these events, noroviruses are deposited onto hands either by the infected person or a caretaker; these viruses are then transferred to a variety of fomites of differing compositions and textures (2, 4, 5). Viral particles aerosolized during vomiting incidents also settle onto fomite surfaces located in the general vicinity of the event (1). The low minimal infectious dose of human norovirus [10 to 100, (1)], coupled with the demonstrated transfer of virus from contaminated surfaces to previously uncontaminated hands, indicates a potential indirect route of transmission that continues to deserve consideration from the public health and research communities.
Upon expulsion from their human host via vomit or diarrhea, noroviruses inevitably lose infectivity and viability over time due to a host of environmental parameters including temperature and humidity. The rate at which viral inactivation occurs has been studied widely for feline calicivirus (human norovirus surrogate) under varying temperatures, humidity, and surface (fomite) types. Following the trend typically seen in laboratory studies, feline calicivirus (non-enveloped) remains viable on surfaces for longer time periods than enveloped viruses (5). In comparison to true enteric pathogens such as hepatitis A virus and murine norovirus; however, feline calicivirus (a respiratory virus) does exhibit a higher rate of inactivation, particularly at extreme pH values (7). In terms of environmental stability, feline calicivirus and murine norovirus displayed similarly low inactivation patterns at 4 °C when dried onto stainless steel surfaces and in suspension (wet conditions); at room temperature; however, murine noroviruses maintained greater viability relative to feline calicivirus under wet and dry conditions (7).
An abundance of disinfection studies have been performed using the aforementioned human norovirus surrogates, with feline caliciviruses comprising a majority of such investigations. Until a viable cell culture system is developed for the human noroviruses that will allow for direct virucidal efficacy evaluations, the surrogate viruses will continue to be employed in their stead.
Selected Pertinent Literature
References
| Virus | Poliovirus |
| Structure | Non-enveloped |
| Family | Picornaviridae |
| Host(s) | Humans |
| Disease(s) Caused | Several manifestations, ranging from mild to life-threatening |
| Symptoms | Fever, headache, stiffness/pain in the back or neck, muscle spasms/tenderness |
| Potential Complications |
Acute poliomyelitis: Flaccid paralysis, loss of reflexes Postpoliomyelitis Syndrome (PPS): Reoccurence of symptoms years after stabilization of acute symptoms |
| Transmission Mode |
Close person-to-person contact, contaminated fomitesFecal-oral, person-to-person contact, water, contaminated fomites |
| Sites of Community Outbreaks | Sewage-contaminated drinking water, poorly sanitized living areas with an unvaccinated population |
Importance of Poliovirus
The polioviruses are the most well-known members of the genus Enterovirus which also includes the echoviruses and Coxsackieviruses. Although poliovirus-related illnesses are believed to have been endemic in various human populations for well over a millennium, the widespread outbreaks most associated with the virus in the United States began during the mid-20th century. Prior to the advent of municipal-level water treatment and thus, readily-available potable water, infants were exposed to wild type polioviruses while still protected by maternal antibodies that provided an initial innate immunity. Repeated environmental exposure(s) further bolstered the host immune response over a lifetime. This natural process of human immunization was interrupted by water treatment, which effectively removed polioviruses and other pathogens of concern from drinking water. The resulting decrease in early childhood exposure led to a surge of susceptible adolescents who were infected during the peak of the pre-vaccine poliomyelitis outbreaks in the U.S. from 1950 to1955. Tens of thousands of children were permanently crippled by polio and several thousand died due to paralysis of the muscles required for breathing. The killed- and live-virus vaccines developments subsequently spearheaded by Jonas Salk and Albert Sabin, respectively, despite their own unique pros and cons, eventually led to eradication of polioviruses from the U.S. in the late 1970s. However, outbreaks of poliomyelitis continue to be recorded around the world in sub-populations of unvaccinated persons (10, 12, 13), with increasing numbers traced to vaccine-derived poliovirus strains in developing countries (9). Therefore, the worldwide eradication of poliovirus remains a top priority for the World Health Organization and a number of aid groups.
There are three known serotypes of poliovirus (PV1, PV2, and PV3) – each are known to only infect humans, with PV1 the most commonly isolated during epidemics (5). Each of the serotypes has a slightly different capsid protein composition; therefore, infection with one serotype does not necessarily impart immunity to the other two serotypes (4). Transmission of polioviruses is by the fecal-oral route similar to the other enteroviruses. Replication initiates in the pharynx and gastrointestinal tract, and the vast majority of infected persons are asymptomatic. Following a transient viremia (virus present in the blood), there is a small chance [<2% (16)] of the virus then invading the central nervous system where motor neurons are subsequently infected, leading to the neurological symptoms associated with poliomyelitis (e.g. flaccid paralysis). Of those cases, there is 5-10% mortality rate due to paralysis of the respiratory muscles. Whether the resulting illness is mild or life-threatening, viruses are shed in the stool for several weeks, resulting in a continuous source of infectious viruses in the environment.
Environmental Viability of Polioviruses and Importance of Disinfection
Polioviruses are characterized by a non-enveloped, nucleocapsid structure, and can remain viable on porous (e.g. paper and cotton cloth) and nonporous surfaces (e.g. glazed tile and aluminum) for up to 30 days with no clear correlation between viability and the presence of organic matter (2). Polioviruses are also more subject to inactivation during the process of desiccation relative to other enteric viruses such as hepatitis A (1). Thus, they are of concern chiefly in the area of water quality due to historical epidemiological aspects (i.e. both waterborne and direct transmission routes). In addition, polioviruses maintain high titer levels and infectivity in aqueous environments, with greater survivability in drinking water and freshwater relative to seawater (6, 8). However, a number of water treatment processes have proven to effectively inactivate polioviruses, including both natural and anthropogenic sources of UV radiation (7, 11) and ozone (15).
For disinfection efficacy evaluations, polioviruses are largely employed as model non-enveloped viruses for suspension-based studies. A recent evaluation of natural and commercially-available disinfectants found that of nine products, only two (which were sodium hypochlorite- or QAC-based) demonstrated a ≥3-log10 reduction of polioviruses in the absence of an organic soil load at 30 seconds and 5 minutes (14). A separate study comparing the susceptibility of polioviruses in the presence of organic soil (20% feces) relative to 0% soil (PBS-only) confirmed the efficacy of sodium hypochlorite in addition to diethylenetriamine. No correlation was found between the presence of organics and viral persistence in suspension, although surface studies may generate different data (1). Further work has demonstrated the anti-viral efficacy of low levels (<2%) of glutaraldehyde against poliovirus in the disinfection of digestive endoscopes used in hospitals and other healthcare settings (3).
References
| Virus | Human Respiratory Syncytial Virus (RSV) |
| Structure | Enveloped |
| Family | Paramyxoviridae |
| Primary Host | Humans |
| Disease(s) Caused | Respiratory disease |
| Symptoms | Runny nose, Coughing, Sneezing, Fever Wheezing |
| Potential Complications | Bronchiolitis, Pneumonia |
| Transmission Mode | Airborne via respiratory droplets produced by coughing and sneezing, potential for indirect transmission via fomites |
| Sites of Community Outbreaks | Hospital wards, Daycare centers, Schools, Hospital wards, Nursing Homes |
Importance of RSV
Human respiratory syncytial virus (RSV) causes respiratory disease in people of all ages. Following an incubation period of several days, most persons experience mild symptoms such as runny nose, coughing, and sneezing, with full recovery within 1 to 2 weeks. Young children (particularly infants), the elderly, and persons afflicted with “high-risk” conditions (e.g. immuno-compromised, chronic heart/lung disease) are particularly susceptible to RSV infections and suffer from potential complications, such as bronchiolitis and pneumonia at higher rates.
RSV is transmitted primarily by way of aerosol droplets released by infected persons upon sneezing and coughing. These infective droplets are then either inhaled directly or settle upon inanimate surfaces (fomites) in the general vicinity. In the latter case (indirect transmission), contact with contaminated surfaces by hands may lead to infection if the individual subsequently rubs their eyes or nose.
The Importance of Disinfection: Survival of RSV on Surfaces and Transmission Potential via Fomites
Respiratory syncytial virus is enveloped in structure and relative to other respiratory viruses both enveloped (e.g. human coronaviruses) and non-enveloped (e.g. rhinoviruses), it loses infectivity more rapidly on inanimate surfaces (2) . However, it has been shown to maintain viability on hard, nonporous surfaces for up to 8 hours. In addition, RSV can survive on skin for up to 20 minutes and transmission has been demonstrated from contaminated fomites to hands (3).
In addition to a relatively higher surface inactivation rate, RSV is also readily rendered non-infective by a number of disinfectant actives. After only a 1-minute contact time, sodium hypochorite (0.1%), chlorhexadine digluconate (1.0%), and benzylakonium chloride (1.0%) demonstrated complete virucidal efficacy against RSV (1). Visual assessment (electron microscopy) of the viruses post-disinfection revealed a loss of virulence factors (i.e. surface glycoproteins) and heavy damage to the viral envelope.
References
| Virus | Rhinovirus |
| Structure | Non-enveloped |
| Family | Picornaviridae |
| Host(s) | Humans |
| Disease(s) Caused | Common cold |
| Symptoms | Runny nose, sore throat, nasal congestion, cough, headache |
| Potential Complications | Secondary bacterial infections (sinusitis, ear infection, etc) |
| Transmission Mode |
Inhalation of aerosols, contact with contaminated fomites |
| Sites of Community Outbreaks | Schools, hospitals, daycare centers, nursing homes |
Importance of the Rhinoviruses
Rhinoviruses are the causative agents of approximately half of all human common colds, with almost officially recognized 100 serotypes (9). The incubation period generally ranges from 1 to 2 days (4), followed by rhinitis (runny nose), nasal congestion, sore throat, cough, and less frequently headaches for symptomatic individuals.
Common cold outbreaks occur worldwide and throughout the year, although in the northern hemisphere most cases are reported from September through April. They tend to occur in locations where persons are in close proximity to one another, such as schools and nursing home facilities (5, 6), with high mortality rates often reported for the latter.
The Importance of Disinfection: Survival of Rhinoviruses on Surfaces and Transmission Potential via Fomites
Unlike the human coronaviruses, which cause approximately 25% of colds (9), rhinoviruses are non-enveloped in structure and grouped phylogenetically with the Picornaviridae family which includes fecal-oral pathogens such as hepatitis A virus. Rhinoviruses do not remain active on surfaces for the extended periods characterized by hepatitis A. However, Rhinovirus 14 has demonstrated viability for >25 hours following inoculation onto hard, nonporous surfaces (stainless steel disks) in the presence of both nasal discharge and tryptose-phosphate broth, with high humidity and low temperature conditions most conducive to viral survival (7).
Coughing and sneezing are hallmark symptoms of the common cold, and the forced projection of nasal fluids (which may contain ~7-log10 of infectious viruses per ml) results in the deposition of infectious viruses on surrounding fomites. Transfer of viruses to the hands occurs by touching these fomites, and self-inoculation has been reported when rhinoviruses were moved from fomites to the nose (mucous membranes) and eyes (1, 2). The disinfection of environmental surfaces therefore becomes of increased importance for the reduction of rhinovirus transmission. Rhinoviruses on stainless steel disks treated for 10 minutes with either a 0.1% o-phenylphenol plus 79% ethanol solution, or bleach (800 ppm free chlorine) were reduced to undetectable levels as detected following attempted transfer to human hands; in contrast, surfaces treated with quaternary ammonium compound and phenolic formulations failed to prevent the transmission of infectious rhinoviruses to the hands (8).
Antimicrobial hand formulations and sanitizers have also been evaluated for efficacy in effort to reduce the transmission of viruses from hands to surfaces and to lower the risk of self-inoculation. Diluted iodine-based (1.0%) solutions in an ethyl alcohol or water base proved most effective for the inactivation of rhinovirus 29 when applied immediately following viral inoculation, with residual activity also observed for up to an hour, while ethyl and benzyl alcohol mixtures proved relatively ineffective (3). Ethanol-based hand sanitizers supplemented with organic acids also proved more efficacious for the elimination of rhinovirus 39 from the hands relative to use of water alone and the combination of water plus soap (10). However, the highly controlled conditions of research studies are not reflective of actual use conditions in natural settings, where people tend towards highly variable behaviors including the volume(s) of antimicrobials applied and the time spent washing/treating the hands.
References
| Virus | Vesicular Stomatitis Virus |
| Structure | Enveloped |
| Genome | Single stranded RNA, negative sense |
| Family | Rhabdoviridae |
| Primary Host | Horses and cattle |
| Disease(s) Caused | Vesicular stomatitis |
| Symptoms | Excessive salivation, oral and teat lesions, lameness |
| Potential Complications | Secondary bacterial infections, weight loss |
| Transmission Mode | Insects, contact with with infected animals, contact with contaminated feed/water |
| Sites of Community Outbreaks | Farms, ranches |
Importance of Vesicular Stomatitis Virus
Vesicular Stomatitis Virus (VSV) is an enveloped member of the Rhabdoviridae family. VSV primarily affects horses and cattle but is also known to infect humans.
VSV causes vesicular stomatitis, a disease that appears similar to foot-and-mouth disease and swine vesicular disease, both foreign to the USA. Symptoms include lesions on or in the mouth, nostrils, and teats. These lesions can break, resulting in oral pain which leads to loss of weight and lameness. In dairy cattle, lesions on the teats can lead to mastitis which results in a drop in milk production. In the absence of secondary infections, animals recover in about 2 weeks. In humans, it causes an acute illness similar to influenza with symptoms including body aches, fever, and lethargy.
VSV can have serious economic consequences. Cases of VSV must be reported to the USDA. Animals from an affected ranch or farm cannot be moved from the premises. This can disrupt training and race schedules for horses and sales for cattle. Affected animals should be isolated in stables. The quarantine lasts for 21 days after the last lesion has healed. Weight loss and mastitis can contribute to a reduction of profit. In one study of 2 dairy farms affected by VSV, the monetary loss was placed at $225,000 during the 2-month period of the outbreak.
Importance of Disinfection: Survival of Vesicular Stomatitis Virus on Surfaces and Potential for Transmission via Fomites.
Transmission of VSV may be initiated by an insect vector. The disease may then spread within a herd by contact with fluid from lesions. Human infection is rare, but proper precautions should still be taken when handling sick animals.
As an enveloped virus, VSV is sensitive to inactivation by environmental factors, such as temperature and humidity. VSV is sensitive to a wide range of disinfectants. A study found that a 0.03% chlorine solution, a 0.0075% iodine solution, and 0.003% quaternary ammonium solution were able to completely inactivate VSV at a 10 minute contact time.
References