ASTM E1052 – Suspension Time-Kill Test for Virus
Get A Testing Quote
Summary of the ASTM E1052 Test
ASTM E1052: Standard Practice to Assess the Activity of Microbicides Against Virucides in Suspension
- The frozen stock test virus is thawed and diluted to a titer of approximately 6-log10 infectious units per 0.1 ml.
- If requested by the Study Sponsor, the test virus is loaded with organic soil to the appropriate level. This is typically done when the test product is intended to be marketed as a one-step disinfectant.
- The EPA requires an organic soil load of 5% for a one-step disinfectant claim.
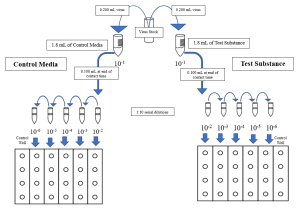
- The test product is prepared to the use-dilution specified by the Study Sponsor, if applicable. An equal volume of buffered saline or cell culture media is prepared to serve as a virus recovery control.
- The prepared inoculum is added to the test product and the virus recovery control media at a ratio of 1 part virus + 9 parts test product (per the Method).
- Upon closure of the study contact time, the test and recovery suspensions are neutralized by a method most suitable for the active ingredient(s) present in the test substance, e.g. dilution into a chemical neutralizer or filtration through a Sephacryl gel column.
- An aliquot of the use-dilution of the test substance is neutralized in an identical manner to the test suspension. This is used to generate the cytotoxicity control to determine the lowest dilution at which, if any, the test substance causes damage to the host cells.
- An aliquot is removed from the cytotoxicity control and inoculated with a low-concentration viral suspension. This is used to generate the neutralization control to confirm the efficacy of the selected neutralization method.
- The neutralized test, recovery, cytotoxicity control, and neutralization control suspensions are serially diluted in the appropriate media. Each dilution is plated in quadruplicate to host cell monolayers in a 24-well tray. Media is added to each well and the host cell-virus system is allowed to incubate for the appropriate time.
- At the close of the incubation time, the assay is scored using standard cell culture methods.
- Each well in the tray is examined under microscope for the presence of cytopathic effects (CPE) of infection. Cytotoxicity control wells are examined for damage caused by the test product. Confirmatory assays are used as necessary.
- The Spearman-Karber method, or another appropriate statistical method, is used to quantify the amount of infectious virus present in the assay.
Virucidal Efficacy Success Criteria
- At least 4-log10 infectious units per 0.1 ml must be recovered from the virus recovery control.
- Complete inactivation of the test virus at all dilutions of the assay.
Strengths of the ASTM E1052 Suspension Time-Kill Method
- Several active ingredient concentrations can be evaluated efficiently and rapidly over various contact times in one study.
- The test method tends to be more reproducible than most hard-surface carrier methods.
Weaknesses of the ASTM E1052 Suspension Time-Kill Method
- The designated mix ratio for testing (1 part virus + 9 parts test product) results in a dilution that may artificially lower product efficacy, particularly for ready-to-use formulations.
- Data generated for formulations against viruses in suspension may not translate to efficacy against dried virus films on hard, nonporous surfaces.
ASTM E1052 Virus Suspension Time-Kill Testing according to U.S. EPA and FDA criteria can be performed at Microchem Laboratory as a simple screen (non-GLP), and under more comprehensive GLP study conditions.
