
SUMMARY
Lineus Medical was founded in 2015 by Spencer Jones, whose experience as a registered nurse gave him a unique perspective on the needs of the medical field. Spencer soon discovered a need, as he noticed that patients were repeatedly losing their peripheral intravenous catheters (IVs) after pulling on the IV tubing. Sometimes on purpose, sometimes by accident, but these pull forces on the IV tubing were causing complications like dislodgement, phlebitis, and infiltration. Spencer thought of the idea of creating a controlled separation between the IV catheter and the IV tubing so that the harmful forces would not harm the IV catheter.
Lineus Medical was formed to remove the “pains”, both physically from patients and financially to hospitals, associated with replacing peripheral IV catheters. They accomplished just this by listening to practitioners and developing a cost-effective device that relieves patients’ pain from IV restarts, saves nurses time, and lowers the overall cost of healthcare.
BACKGROUND
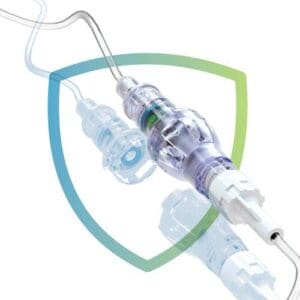 Dangerous complications such as infiltration, phlebitis, and dislodgement can be caused by harmful forces on peripheral IV lines and may ultimately lead to an IV restart. Lineus’ device, SafeBreak Vascular, is the first of its kind in a brand-new classification of devices called Force-Activated Separation Devices. As these forces cause IV-line tension, SafeBreak intentionally separates to protect the patient and the peripheral IV catheter. Upon separation, a valve on the patient side closes to prevent blood loss and a valve on the IV end closes to prevent expensive, and potentially hazardous, fluid spills. SafeBreak allows for fewer peripheral IV mechanical complications requiring IV replacement and ultimately prevents patients from having additional painful needle sticks.
Dangerous complications such as infiltration, phlebitis, and dislodgement can be caused by harmful forces on peripheral IV lines and may ultimately lead to an IV restart. Lineus’ device, SafeBreak Vascular, is the first of its kind in a brand-new classification of devices called Force-Activated Separation Devices. As these forces cause IV-line tension, SafeBreak intentionally separates to protect the patient and the peripheral IV catheter. Upon separation, a valve on the patient side closes to prevent blood loss and a valve on the IV end closes to prevent expensive, and potentially hazardous, fluid spills. SafeBreak allows for fewer peripheral IV mechanical complications requiring IV replacement and ultimately prevents patients from having additional painful needle sticks.
TESTING AND COLLABORATION
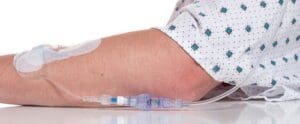
Lineus Medical has collaborated with the Medical Device Efficacy Team at Microchem since 2019 to ensure their product met the FDA’s strict standards for intravascular medical device accessories. Lineus Medical worked closely with Microchem’s skilled Analysts to create customized protocols and procedures to capture critical data to meet their regulatory needs. Microchem was able to collaborate with Lineus Medical to design and perform testing that was just as unique and successful as their SafeBreak Vascular device.
STORY OUTCOME
 In May of 2021, the FDA officially cleared SafeBreak for sale in the United States. Being a De Novo clearance as opposed to a 510(k), SafeBreak Vascular became the first FDA-cleared Force-Activated Separation Device of its kind. A clinical study using SafeBreak Vascular at Harford Hospital in Hartford, CT was successfully conducted and published in the March 2022 edition of The Journal of Infusion Nursing. This randomized clinical study demonstrated that the use of SafeBreak reduced overall IV complications requiring an IV restart by 46% while causing no additional delays in therapy for patients. Microchem is happy to be a part of the process involved in creating these groundbreaking technologies.
In May of 2021, the FDA officially cleared SafeBreak for sale in the United States. Being a De Novo clearance as opposed to a 510(k), SafeBreak Vascular became the first FDA-cleared Force-Activated Separation Device of its kind. A clinical study using SafeBreak Vascular at Harford Hospital in Hartford, CT was successfully conducted and published in the March 2022 edition of The Journal of Infusion Nursing. This randomized clinical study demonstrated that the use of SafeBreak reduced overall IV complications requiring an IV restart by 46% while causing no additional delays in therapy for patients. Microchem is happy to be a part of the process involved in creating these groundbreaking technologies.
