Liquid hand sanitizers – mostly alcohol-based gels – have enjoyed an explosion in popularity in the last 10 years.
If you have traveled by airplane or set foot in a classroom in the US lately, chances are you have seen hand sanitizers in use.
Hand sanitizers do not serve as a replacement for thorough handwashing. Instead, they are thought to bring consumers some of the benefits of handwashing when handwashing is not practical.
The relationship between hand sanitizer use and reduced illness has not been firmly established by epidemiological studies, but several laboratory studies suggest hand sanitizers help to prevent infections by killing transient pathogenic bacteria.
Handwashing and hand sanitizers reduce microbial populations in different ways. Handwashing – whether done with “antibacterial” soap or plain soap – physically removes microorganisms from the skin, literally washing the live microbes down the drain. Hand sanitizers reduce levels of microorganisms by killing them chemically, just like disinfectants kill germs on environmental surfaces.
The magnitude of the effect of handwashing is mainly a function of wash time and soap usage. Washing hands without soap is much less effective. Effectiveness from hand sanitizers is best when a large volume of product is applied to the hands. Applying a large volume of hand sanitizer ensures excess active ingredient and extends the period of chemical activity before the hand sanitizer evaporates.
Unlike disinfectants, which may be left practically on surfaces for up to about 5 minutes, hand sanitizers must do their job within a brief period of time to produce the necessary effect. The reality is that most people just won’t tolerate wet hands for more than about 30 seconds. Accordingly, Microchem Laboratory believes that 30 seconds – maybe one minute in special cases – should be the contact time limit for laboratory testing of hand sanitizers.
Hand sanitizers may be powered by a number of different active ingredients, but have you ever noticed that most hand sanitizers use alcohol as the active ingredient? That is largely a result of how they are regulated.
Regulation of Hand Sanitizers by FDA
Hand sanitizers are regulated in the USA by the Food and Drug Administration (FDA) as drugs. In 1994, the FDA published a document called the “Tentative Final Monograph for OTC Healthcare Antiseptic Drug Products.” It is commonly known in the industry as the TFM. The document, though “tentative,” serves as a road map to testing requirements and covers all sorts of antimicrobials meant to be applied to skin, including hand sanitizers. FDA is interested in finalizing the monograph, but it is not expected to be finalized any time soon.
Companies interested in marketing a hand sanitizer in the United States will benefit from becoming familiar with the FDA Tentative Final Monograph. Sections of the FDA Tentative Final Monograph follow, with excerpts hightlighted and discussed in further detail. Remember, the tables and sections discussed below come from the tentative final monograph, so there’s room to customize and streamline studies for submission to regulatory agencies.
FDA TFM Excerpt #1: FDA-Known Topical Antimicrobials, Data Requirements
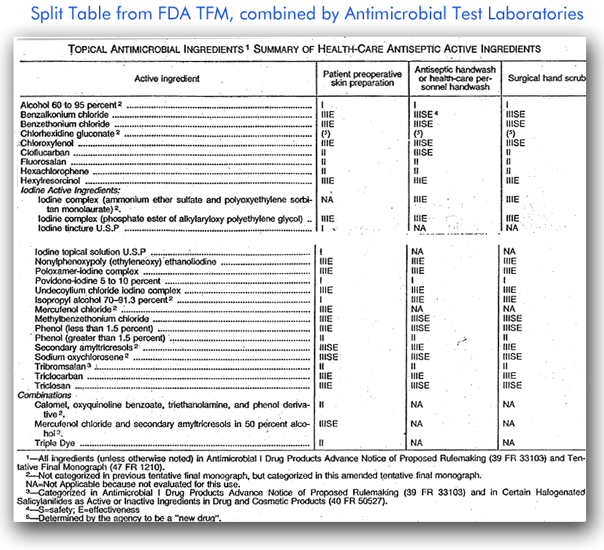
The table above lists various chemicals which may be used to power various types of topical antimicrobials and provides information about whether safety and efficacy testing are required. The Roman numerals in the table identify the product category, and the presence of “S” and/or “E” indicates whether safety and/or efficacy testing is required.
If there is one thing to learn from the table above, it is that a product may or may not need efficacy or safety testing, depending on its active ingredient. For example, as Microchem Laboratory interprets the table, 60-95% ethanol hand sanitizer formulations do not require efficacy testing though it is a good idea for companies to do confirmatory efficacy testing, even if not required by FDA.
If efficacy testing of your product is required, FDA’s suggestions per the TFM are detailed in the page below. If the active ingredient your company wishes to use to make a hand sanitizer is not listed, then it will likely be considered a “new drug” by FDA and subject to a New Drug Application (NDA). In rare instances, an active ingredient not listed in the monograph may be permitted in commerce if it can be shown it was used as a hand sanitizer prior to certain regulations taking effect (grandfathered).
FDA TFM excerpt #2: Page 31444, Detailing Tentative Efficacy Test Guideline

As the highlighted sections of the document show, the Tentative Final Monograph suggests testing across a broad array of bacteria and fungi using both MIC and time-kill methods, which are the twoin vitro;components of the efficacy testing process. It also indicates that a clinical “glove test” is performed, typically upon completion of the MIC and Suspension Time-Kill studies. The in vivoportion of efficacy testing is carried out using a bacterium called Serratia marcescens, which is inoculated onto the hands of volunteers and then recovered by massaging the hand in a broth filled, sterile glove.
The tentative final monograph is vague with regard to in vitro performance criteria. It suggests myriad MIC assays and lays out criteria for suspension time-kill tests using product tested at a considerable dilution over lengthy contact times. The relevance of this information to hand sanitizer performance is questionable. Microchem Laboratory usually recommends a series of tests for Class III hand sanitizers that differs from that laid out by the TFM.
Microchem thanks you for taking the time to learn about hand sanitizers!
