Layperson’s Summary
To do an MIC, one inoculates the test substance with an invisible but high number of microorganisms, then observes the mixture of microorganisms and test substance to see if it changes from clear to cloudy. If it turns cloudy, that means microorganisms have grown to high levels, and the test substance is not inhibitory to them at that particular dilution.
Test wells that remain clear after incubation may contain the original low-level inoculum of viable microorganisms, or the microbes could all have been killed by the antimicrobial agent. Those two outcomes cannot be differentiated visually. For that reason, scientists use MIC assays as indicators of an antimicrobial agent’s inhibitory activity rather than biocidal activity.
Labeled Photo of Microtiter MIC Test – 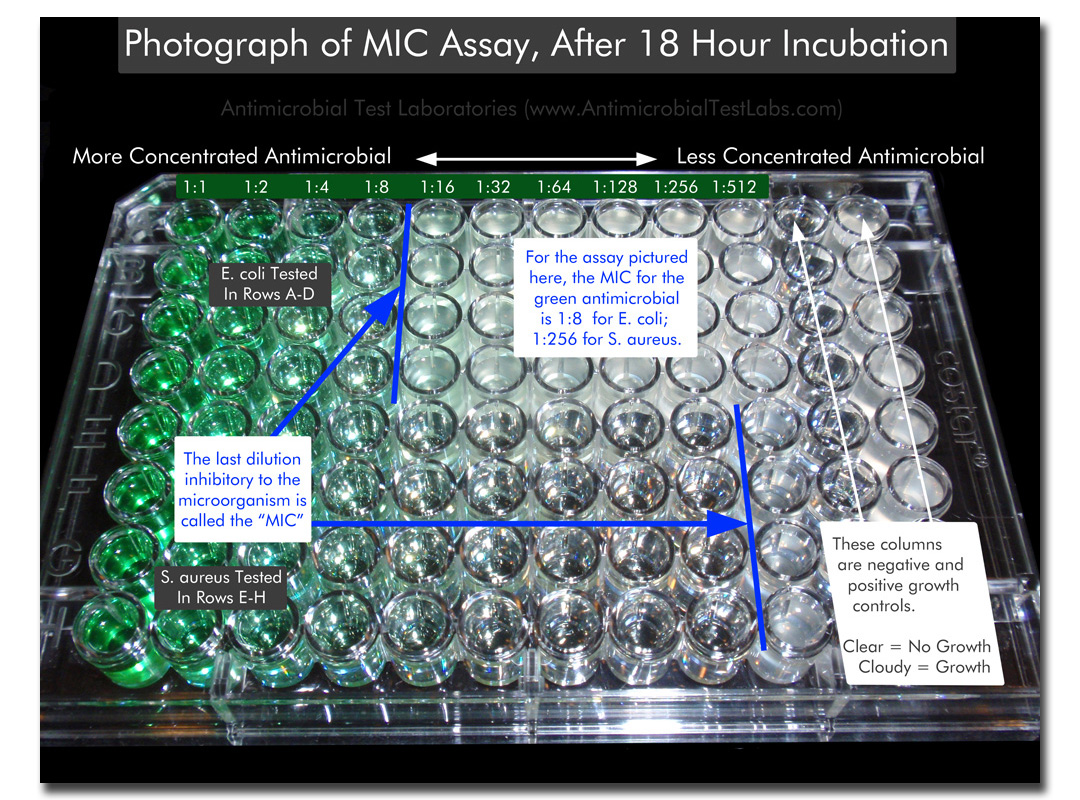
Labeled Photo of Standard MIC Test – 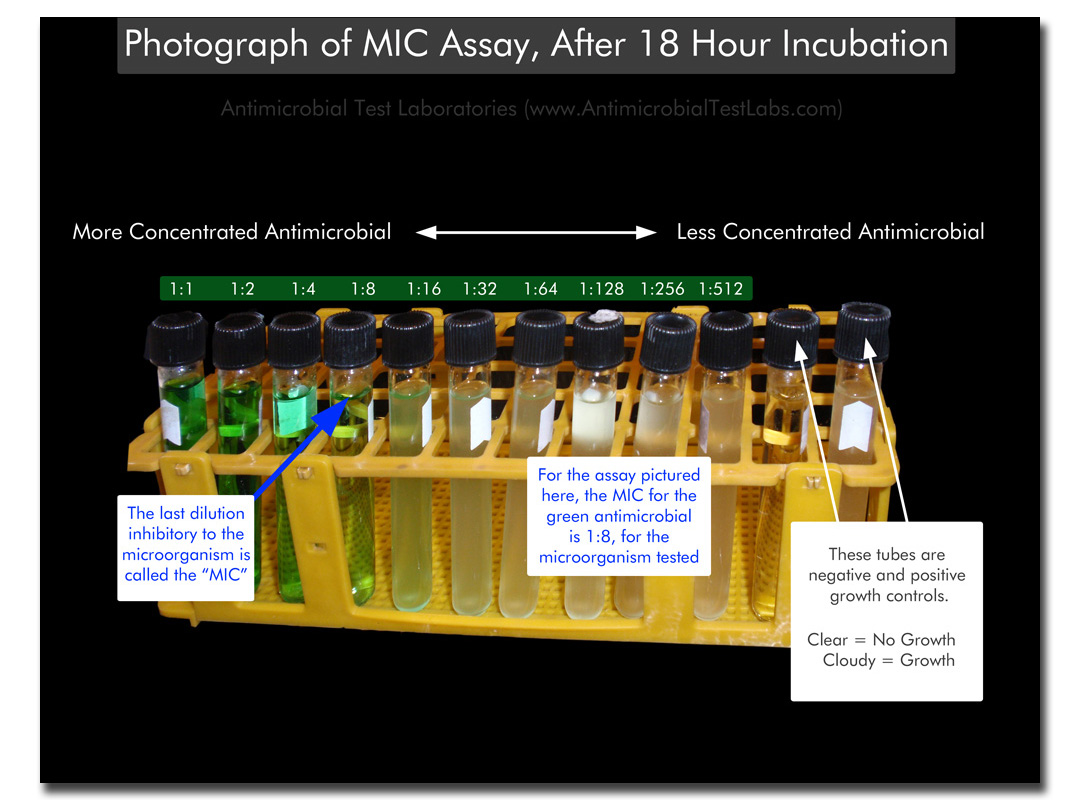
Summary of the Minimum Inhibitory Concentration Test (MIC Test):
- A pure culture of a single microorganism is grown in Mueller-Hinton broth or other broth as appropriate.
- The culture is standardized using standard microbiological techniques to have a concentration of very near 1 million cells per milliliter. The more standard the microbial culture, the more reproducible the test results.
- The antimicrobial agent is diluted a number of times, usually 1:1, through a sterile diluent (typically Mueller-Hinton broth).
- After the antimicrobial agent has been diluted, a volume of the standardized inoculum equal to the volume of the diluted antimicrobial agent is added to each dilution vessel, bringing the microbial concentration to approximately 500,000 cells per milliliter.
- The inoculated, serially diluted antimicrobial agent is incubated at an appropriate temperature for the test organism for a pre-set period, usually 18 hours. The more consistent the incubation period, the more reproducible the test results.
- After incubation, the series of dilution vessels is observed for microbial growth, usually indicated by turbidity and/or a pellet of microorganisms in the bottom of the vessel. The last tube in the dilution series that does not demonstrate growth corresponds with the minimum inhibitory concentration (MIC) of the antimicrobial agent.
Strengths of the Minimum Inhibitory Concentration Test (MIC Test):
- The MIC test is relatively straightforward and easy to prepare for and execute, which naturally enhances reproducibility.
- The MIC test can be done on a very small scale without using too much antimicrobial agent. This is important for experimental antimicrobials, such as biologically synthesized antimicrobial peptides.
- The MIC test is an easy way to test the antimicrobial attributes of a formulation across many different parameters, such as across microbial species or surfactant blends.
- Because little preparation is required for the minimum inhibitory concentration testing, test turnaround times can be kept low.
Weaknesses of the Minimum Inhibitory Concentration Test (MIC Test):
- Minor variations in the MIC test parameters can have major impacts on the apparent MIC. For example, extended incubation will make the MIC appear to be higher, and lower inoculum concentrations will make the MIC appear to be lower.
- Results from MIC studies must be evaluated in the appropriate context. In the tube corresponding to the MIC, microorganisms were merely prevented from growing and not necessarily killed – there could still be 500,000 viable cells in that dilution vessel just waiting to grow should the antimicrobial agent become chemically neutralized!
MIC tests are an important and unique part of Microchem Laboratory’s portfolio of testing services. MIC tests are especially appropriate if liquid antimicrobial agents will be used to inhibit the growth of microorganisms in some context.
MIC tests can be followed by tests for germicidal (microbe-killing) activity with a few extra steps, in a test called a Minimum Bactericidal Concentration test (MBC)
